Recently, Professor Su Yaorong's team from our institute published their latest research findings titled "Electrostatic self-assembly of g-C3N4/AgInS2 heterostructures with electronic localization at sulfur site for enhanced CO2 photoreduction performance" in the high-level international academic journal *Journal of Colloid and Interface Science* (a first-tier journal of the Chinese Academy of Sciences with an impact factor of 9.4). Shenzhen Technology University's School of New Materials and New Energy was the sole completing institution. Associate Professor Meng Aiyun and master's student Yang Peiyi are co-first authors, while Professor Su Yaorong and Associate Researcher Zhong Wei are co-corresponding authors.
In the context of global warming and energy crises, how to efficiently convert carbon dioxide (CO₂) into renewable fuels has become a research focus. Traditional graphite phase nitrogen carbide (g-C₃N₄) photocatalysts suffer from limitations such as weak CO₂ adsorption capacity, narrow light absorption range, and rapid charge recombination, which restrict their photocatalytic performance. Professor Su Yaorong's team innovatively developed a g-C₃N₄/AgInS₂ heterojunction photocatalyst, achieving efficient material compositing through electrostatic self-assembly technology, providing new insights for CO₂ photocatalytic reduction.
The research team utilized the energy level difference between g-C₃N₄ and AgInS₂ to construct a tight interfacial contact through electrostatic adsorption, facilitating the directed transfer of electrons from g-C₃N₄ to AgInS₂, forming electron-rich sulfur sites (S(2+δ)⁻). Theoretical simulations and experiments show that this structure significantly enhances CO₂ adsorption capacity (with an adsorption energy of-1.58 eV) and broadens light absorption to the near-infrared region (with an absorption edge reaching 1000 nm). The g-C₃N₄/AgInS₂ heterojunction also greatly improves charge separation efficiency, increasing the photocurrent density by 14 times compared to pure g-C₃N₄. The optimized catalyst achieves a CO production rate of 121.08 μmol·h⁻¹·g⁻¹ under visible light for CO2 reduction, more than doubling that of a single material, with no performance degradation over 5 hours of continuous reaction. In-situ infrared spectroscopy reveals that CO₂ converts to CO via the *COOH intermediate, with the electron-rich nature of the sulfur site directly driving carbon-oxygen bond activation, promoting the adsorption and conversion of CO2.
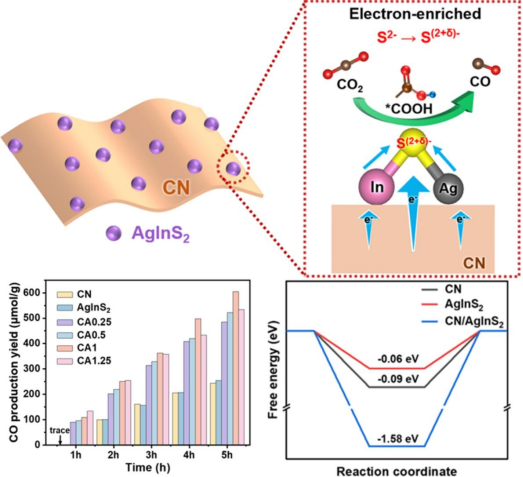
Figure 1 Schematic diagram of CO2 reduction performance and mechanism of g-C₃N₄/AgInS₂ heterojunction photocatalyst.
This study provides a new idea for the development of high-performance CO2 reduction photocatalysts by regulating the electron density of active sites through heterojunction design. This work has both environmental treatment and energy conversion value, and is expected to facilitate the implementation of CO₂ conversion technology and accelerate the realization of carbon neutrality goals.
This work was supported by the National Natural Science Foundation of China, Guangdong Provincial Basic and Applied Basic Research Fund, Guangdong Provincial Department of Education, Shenzhen Municipal Science and Technology Innovation Commission, Shenzhen Key Laboratory of Super Diamond and Functional Crystal Application Technology, Hubei Provincial Key Laboratory of Pollutant Analysis and Resource Recovery Technology Open Fund.
Original link: https://www.sciencedirect.com/science/article/pii/S0021979724030960?via%3Dihub